
Repeat Step 7 with the remaining olive oil standard samples.ĩ.

To save your data, select "Store Latest Run" from the Experiment menu.Ĩ. Review the graph to identify the peak absorbance values.
#Logger pro absorbance full#
Click "collect." A full spectrum graph of the olive oil will be displayed. Empty the blank cuvette and rinse it twice with small amounts of extra virgin olive oil.įill the cuvette ¾ full with the olive oil and place it in the spectrometer.ī. Conduct a full spectrum analysis of an olive oil sample:Ī. Click "Finish Calibration", and then click "OK."ħ. Align the cuvette so that theĬlear sides are facing the light source of the spectrometer. Place the blank in the cuvette holder of the spectrometer. seconds for the device to warm up." After 60 seconds, the message changes to: "Warm up complete."Ĭ. Message appears in the Calibrate dialog box: "Waiting. Open the Experiment menu and select Calibrate → (Spectrometer). Prepare a blank by filling an empty cuvette ¾ full of distilled water.ī. To set up the spectrometer, open the Experiment menu and select Connect Interface → Spectrometer → Scan for Spectrometers.Ī. Obtain small volumes of the three standard olive oils to be tested.ĥ. Start the Logger Pro 3.4.6 program on your computer.Ĥ. Use a USB cable to connect a Vernier Spectrometer to a computer.ģ.
#Logger pro absorbance software#
The procedure used to conduct the lab is as follows: (Vernier Software and Technology, Spectroscopy with Vanier, 2006, P.
#Logger pro absorbance how to#
To get started, show students during a demonstration how to complete the following tasks: setting up the spectrometer, using Logger Pro 3.4.6 program on the computer or Lab Quest, calibrating the spectrometer, conducting the spectrum analysis of the olive oil samples, saving the experimental files on the computer, and saving and printing the absorbance Vs wavelength graphs. This would require a couple of days of exploratory and simple canned lessons. Before beginning this lab, I would highly suggest that students become familiar with both Lab Quest and the spectrometer hardware and software. The lesson will be introduced during the properties unit in chemistry and physical science. An extension would be to soak another type of green leaved plant in the isopropyl alcohol to analyze the chlorophyll absorbance and wavelength. The "unknown" should be either one of the three types of olive oils they have already analyzed. The final activity is for each group to identify an "unknown" determined by the teacher. Students should be able to differentiate the different types of olive oil absorbance Vs wavelength on the final graphs. It would be encouraged to complete a demo and show students what the expected results graph will look like and discuss absorbance and concentration before the lab begins. You should expect a few days to get students acclimated to the Varnier spectrometer, lecture, and completing the lab and analysis. This lab requires an extensive pre-lab component and demonstration by the teacher.
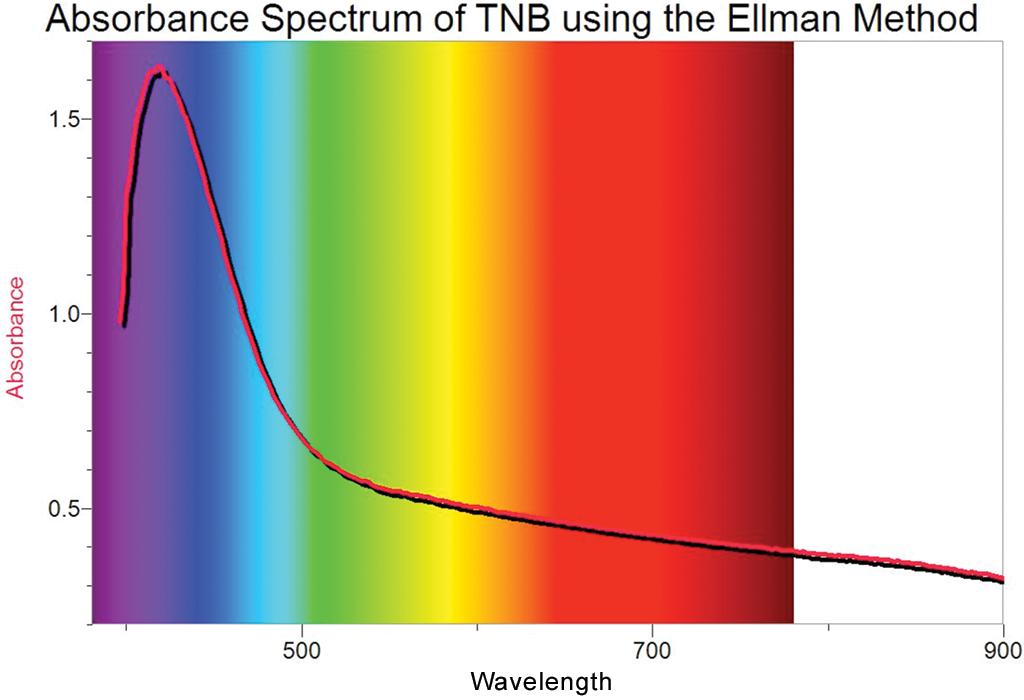
I would suggest using olive oil or other green leaved plants to extract olive oil samples. Special equipment includes the Vernier Spectrometer, computers, cuvettes, Beral pipets, three different types of olive oil, distilled water, and isopropyl alcohol. Students are divided up into groups of four. There are 20-35 students per class on any given day and class session. This activity could include students in grades 9-12. Vocabulary words that students will learn through this activity are, spectrometer, absorbance, absorbance spectrum, light spectrum, chlorophyll, cuvette, Beral pipet, and nanometer. Lastly, students will learn how to use the Vernier Spectrometer (V-SPEC) to measure the absorbance of the olive oil samples over the visible-near infrared (NIR) light spectrum between 380 and 950 nanometers. Students will realize that in the world of chemistry, specifically, chlorophyll, that all greens are not the same. They will determine that different sources of chlorophyll in olive oil have different ratios of these absorbance peaks, which create various shades of green. Students will examine combinations of these wavelengths which are green to the human eye. From this activity, students will understand the absorbance spectrum, in the visible light range, of chlorophyll creates three yellow absorbance peaks at 413, 454, and 482 nanometers, and two blue absorbance peaks at 631 and 669 nanometers.


 0 kommentar(er)
0 kommentar(er)
